
VCAS™ White Pozzolans
Portland Cement and Pozzolans
Custom-engineered, high performance, pozzolanic mineral additives for use in white cement, mortar, and concrete products.
Technical Background for the Effective Use of VCAS™ Pozzolans in Portland Cement Concrete.
Background on Portland Cement
Cement Manufacture
Portland cement is manufactured by ball milling raw materials such as limestone, clay or shale, and an iron source, then firing in a rotary kiln at about 1400°-1500°C. The resultant “clinker” is then cooled, and ground again in a bill mill with gypsum (to retard set) to about 90-95% minus 325 mesh, or 10-15 microns in median particle size. Since cement plants use local raw materials whenever possible, cement chemistry will vary somewhat based on local mineral compositions. Efforts are made to keep the alkali components (Na2O and K2O) less than 1% to minimize deleterious reactions with reactive aggregates. White Portland cement is manufactured similarly to gray Portland, but efforts are made to reduce iron and total alkalis for better color and lower reactives. Typical ASTM C150 Portland cements are made up of 50-70% C3S (tricalcium silicate), 15-20% C2S (dicalcium silicate); 5-10% C3A (tricalcium aluminate); 5-15% C4AF (ferrite phase); and 3-5% gypsum (calcium sulfate).
Hydration of Portland Cement
When cement hydrates, the principal products generated are 50-70% C-S-H (calcium silicate hydrate); 10-15% ettringite (calcium sulfoaluminate); and 20-25% CH (calcium hydroxide or ”lime”). The C-S-H is the strength building binder for concrete, whereas the CH has no strength building properties and leads to efflorescence and poor chemical resistance.
Replacing 25% of the cement with a pozzolan reduces the formation of CH (by dilution); plus the pozzolan reacts with the remaining CH to form additional C-S-H binder. This strengthens the concrete, reduces permeability, and adds durability (see later).
Chemistry of Hydration – It is assumed that each compound hydrates independently of others in Portland cement. This is not completely true because interaction between hydrating compounds will affect the mix.
Calcium Silicates – The hydration reaction of the two calcium silicates, which make up the largest percent of Portland cement, are similar.
The principle products are: (a) calcium silicate hydrate, poorly crystalline material of extremely small particle size; and (b) calcium hydroxide, a crystalline material. The reaction can be measured by the rate of heat generation.
-
Stage 1 – Rapid heat generation (15 min.) – on mixing with water, calcium and hydroxide ions are released from the surface of the C3S; pH rises to a very alkaline solution. When the calcium and hydroxide reach critical concentrations, crystallization of CH and C-S-H begins. Early chemical reactions are temperature dependent.
-
Stage 2 – Dormant period – causes cement to remain plastic (2-4 hours). The reaction slows. CH crystallizes from the solution, C-S-H develops on the surface of the C3S and forms a coating. As the thickness increases, the time it takes water to penetrate the coating increases, thus the rate of reaction becomes diffusion controlled. C2S hydrates at a slower rate because it is a less reactive compound.
-
Stage 3 – Acceleration period – Critical concentration of ions is reached and silicate hydrates rapidly, maximum rate occurs at this stage. Final set has passed and early hardening begins (4-8 hours).
-
Stage 4 – Deceleration – rate of reaction slows; completely diffusion dependent reaction.
-
Stage 5 – Steady state – constant rate of reaction (12-24 hours). Temperature has little effect on hydration atthis point.
Tricalcium Aluminate – Hydration of C3A occurs with sulfate ions supplied by dissolved gypsum. The result of the reaction is calcium sulfoaluminate hydrate, called “ettringite” after a naturally occurring mineral.
-
If the supply of sulfate from the gypsum is exhausted before the C3A is completely hydrated, a second reaction can occur. The product of this reaction is monosulfoaluminate. This reaction may occur before the formation of the ettringite if the reaction of C3A and the sulfate ions is faster than the gypsum will allow.
-
The ettringite decreases the reaction by forming a diffusion coating around the C3A similar to the reaction of C3S. The coating can be broken down by the conversion to monosulfoaluminate.
-
If the monosulfoaluminate is exposed to another source of sulfate ions, then a new reaction will occur forming more ettringite. This new formation causes volume to increase and leads to tensile cracking. This tendency is one form of sulfate attack of Portland cements.
-
In the absence of sulfates, C3A reacts with water to form two unstable calcium aluminate hydrates which later convert to hydrogarnet. A pure C3A paste will not develop significant strength.
Ferrite Phase (C4AF) – forms the same hydration products as C3A, with or without gypsum. The reaction is slow and is decreased further by gypsum. If the iron oxide content is increased, the reaction is slower. Experience has shown that cements low in C3A and high in C4AF are more sulfate resistant. The conversion from ettringite to monosulfoaluminate is inhibited by the presence of the iron component.
The rate of hydration is on the order of C3A > C3S > C4AF > C2S. Reactions for even identical compounds may vary due to: (a) fineness, (b) rate of cooling of clinker, and (c) impurities.
Properties of the Hydration Products
Some general comments on the properties of hydration products affecting the overall behavior of the cement. C-S-H,
Calcium Silicate Hydrate – low degree of crystallinity; the exact chemical composition is variable. The ratio of C/S varies between 1.5 and 2.0 and depends on many factors, such as temperature, w/c ratio, impurities, etc. Likewise, the water content varies considerably.
Because of the low crystallinity, C-S-H develops very small irregular particles and consequently a very high surface area. In general, the surface area of the hydrated cement is about 1000 times larger than the unhydrated cement. Therefore, the increase in surface area greatly influences the physical properties of the C-S-H hydrate.
Calcium Hydroxide – a well understood hexagonal crystalline material. The crystals are much larger than C-S-H particles and are sometimes visible to the naked eye.
Calcium Sulfoaluminate (Ettringite) – These hexagonally-shaped prism crystals are considerably longer than CH crystals. Large clusters of ettringite needles may be visible in concrete affected by sulfate attack. Monosulfoaluminate tends to form very thin, hexagonal plates.
Microstructure of Hydrated Cement Pastes
The development of cement microstructure relates to the five chemical stages described earlier.
C-S-H – the largest component of the cement paste (50-70%) and is the most important component in the hydration process. The amount of C-S-H coating on a C3S grain is very small during state 2 of hydration and increases rapidly in stage 3. The spines of the forming C-S-H radiate outward from each grain with the bulk of the material below the spines. As the C-S-H hydrates further, the coating thickness grows, forcing the outward spines of adjacent particles to interlock to form solid bonds. As hydration continues, the intermeshed spines contribute to an increase in the undercoating of C-S-H growth. The effect is to bond the cement grains together with the C-S-H coating.
CH – constitutes 20-25% of the binder volume. In the acceleration stage, CH grows in the capillary pore space. CH will only grow in free space; on encountering another CH crystal it will grow in another direction; also it will grow completely around a hydrating cement grain. The latter effect gives the CH a larger apparent volume in cement pastes than it would have as a pure crystal.
Calcium Sulfoaluminate – a small component of cement pastes (10-15%) having little effect on microstructure. Young spiny ettringite crystals grow into capillary space and later convert to flat monosulfoaluminate crystals. There will be unhydrated residues in the cement paste, mainly caused by calcium hydroxide, even in very matured hydrated pastes.
Porosity – a major component of microstructure which will influence paste properties. Pore size distribution is difficult to measure. Many tests require drying, which affects the pore structure. There are two classifications of pore sizes:
-
Capillary pore – space formed between hydrating gains.
-
Gel pores – very small spaces in the C-S-H coating. Constitutes the bulk of porosity in a cement paste.
Properties of Hydrated Cement Pastes
Hydration products have lower specific gravities and larger specific volumes than their parent cement compounds.
Therefore, every hydration reaction is accompanied by an increase in solid volume.
-
Calcium Silicates – hydration of these materials is not accompanied by an increase in volume. Recall, these crystals will only occupy free space. If this space is filled, the growth or hydration will stop.
-
Calcium Aluminate – The hydration product of this material (ettringite) will continue to form even when a solid surface is encountered. Since there is no volume in which the crystal can grow, internal pressures develop.
Volume change is directly related to porosity. It is possible to calculate pore space by measuring the loss of evaporable water and nonevaporable water. The evaporable water describes water held in capillary and gel pores. These can be determined by controlled heating, ideally using thermogravimetric analysis capable of reaching high temperatures (1000°C). T.C. Powers developed several empirical relationships for degree of hydration based on the amount the two types of water described above.
wn = 0.24a g/g of original cement Where, a = degree of hydration and wn = nonevaporable water wg = 0.18a g/g of original cement Where, wg = gel water or evaporable water Other relationships for volume of hydration products and porosity are available. Based on these, a minimum water/cement ratio relationship for complete hydration can be formed.
wmin = (wn + wg) g/g of original cement (w/c)min = 0.42a Therefore, for complete hydration, the w/c ratio should not fall below 0.42. However, complete hydration is not required for high ultimate strength. This means that paste with low w/c rations will self-desiccate unless external water is added. Generally, this is not a problem in the field.
CH – constitutes 20-25% of the binder volume. In the acceleration stage, CH grows in the capillary pore space.
CH will only grow in free space; on encountering another CH crystal it will grow in another direction; also it will grow completely around a hydrating cement grain. The latter effect gives the CH a larger apparent volume in cement pastes than it would have as a pure crystal.
Calcium Sulfoaluminate – a small component of cement pastes (10-15%) having little effect on microstructure. Young spiny ettringite crystals grow into capillary space and later convert to flat monosulfoaluminate crystals. There will be unhydrated residues in the cement paste, mainly caused by calcium hydroxide, even in very matured hydrated pastes. Porosity – a major component of microstructure which will influence paste properties. Pore size distribution is difficult to measure. Many tests require drying, which affects the pore structure. There are two classifications of
pore sizes:
-
Capillary pore – space formed between hydrating gains.
-
Gel pores – very small spaces in the C-S-H coating. Constitutes the bulk of porosity in a cement paste.
Properties of Hydrated Cement Pastes
Hydration products have lower specific gravities and larger specific volumes than their parent cement compounds. Therefore, every hydration reaction is accompanied by an increase in solid volume.
-
Calcium Silicates – hydration of these materials is not accompanied by an increase in volume. Recall, these crystals will only occupy free space. If this space is filled, the growth or hydration will stop.
-
Calcium Aluminate – The hydration product of this material (ettringite) will continue to form even when a solid surface is encountered. Since there is no volume in which the crystal can grow, internal pressures develop.
Volume change is directly related to porosity. It is possible to calculate pore space by measuring the loss of evaporable water and nonevaporable water. The evaporable water describes water held in capillary and gel pores. These can be determined by controlled heating, ideally using thermogravimetric analysis capable of reaching high temperatures (1000°C). T.C. Powers developed several empirical relationships for degree of hydration based on the amount the two types of water described above.
wn = 0.24a g/g of original cement
Where, a = degree of hydration and wn = nonevaporable water
wg = 0.18a g/g of original cement
Where, wg = gel water or evaporable water
Other relationships for volume of hydration products and porosity are available. Based on these, a minimum water/cement ratio relationship for complete hydration can be formed.
wmin = (wn + wg) g/g of original cement
(w/c)min = 0.42a
Therefore, for complete hydration, the w/c ratio should not fall below 0.42. However, complete hydration is not required for high ultimate strength. This means that paste with low w/c rations will self-desiccate unless external water is added. Generally, this is not a problem in the field.
Pozzolans in Concrete
A pozzolan is broadly defined as an amorphous or glassy silicate or aluminosilicate material that reacts with calcium hydroxide formed during the hydration of Portland cement in concrete to create additional cementitious material in the form of calcium silicate and calcium silicoaluminate hydrates. The first pozzolans were used by the Romans to make cement from burned limestone and Santorum earth from volcanic eruptions. These ancient concrete mixes were extremely durable and many architectural elements survive today. They underline the fact that one of the compelling reasons for incorporating pozzolans in concrete today is to improve quality and to extend service life by enhancing the durability of this ubiquitous construction material.
To function properly, pozzolans must be amorphous or glassy and generally finer than 325 mesh (45 microns) in particle size. Finer particle sizes generally have greater reactivity, meaning they more quickly convert to supplementary cementitious material, helping in early strength development as measured by standardized tests such as ASTM C618/C109.
Pozzolans can continue to react in concrete for many years, further strengthening the concrete and making it harder and more durable during its service life. Pozzolans also serve to densify and reduce the permeability of concrete, which helps to make it more resistant to deterioration and swelling associated with various exposure conditions.
Types of Pozzolan
Pozzolans commonly used in modern concrete construction include coal fly ash (aka pulverized fuel ash or PFA), ground granulated blast furnace slag, silica fume, and metakaolin (calcined clay). The common feature of all these pozzolans is that they are silicates or aluminosilicates that have been converted to amorphous or glass phases in a high temperature furnace or combustion chamber, followed by rapid cooling or quenching under various conditions. The amorphous or glassy form allows the silicates to react readily as the concrete cures. For use in modern cement and concrete applications, pozzolans must be low in alkalis (Na2O and K2O), which cause longterm durability problems in concrete by expansion due to the alkali-silica reaction (ASR). Chemically, pozzolans are comprised principally of oxides of silicon, aluminum and calcium. The composition of the common pozzolans, including VCAS™ pozzolans, relative to Portland cement are compared in the ternary diagram (CaO-SiO2-Al2O3) below.
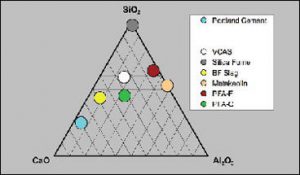
Ternary diagram (CaO-SiO2-Al2O3) showing the composition of VCAS™ pozzolans relative to Portland cement and the common pozzolans.
The Pozzolanic Reaction
There are several steps involved in the pozzolanic reaction in concrete. As Portland cement reacts with water, the tricalcium silicate (C3S) and dicalcium silicates (C2S) react to form calcium silicate hydrates (C-S-H), largely responsible for strength development, together with calcium hydroxide, Ca(OH)2 (often referred to a “lime”). At the same time, the alkalinity of the water (now referred to as pore fluid) increases to pH 13 or higher. This combination of events provides ideal conditions under which the pozzolan can react. The high pH first causes the silicate network structure of the pozzolan to break down to smaller units, which then react with the calcium hydroxide to form more calcium silicate hydrate binder. The net effect is that the calcium hydroxide in the concrete — which itself has no strength-forming properties and is also a potential site of weakness for certain forms of chemical degradation — is converted by the pozzolan to additional C-S-H binder which is deposited in pore spaces. This leads to a general densification of the cement matrix, which contributes to increased strength, reduced permeability, and increased long-term durability. More details on the hydration of Portland cement are given in the Appendix.
Portland cement requires only about 25% water to completely react to form concrete. However, many concrete mixes employ larger percentages of water content to allow the mix to flow properly into the formwork or to achieve proper workability. This excess water inevitably introduces void spaces in the concrete that make the concrete porous and provide conduits for the passage of water and aggressive solutions. It is therefore easy to understand that the water-to-cement (w/c) ratio is a major controlling factor in the strength potential of a given concrete mix design: low w/c ratios are associated with higher strengths; whereas high w/c ratios are associated with lower strengths. The conversion of the calcium hydroxide to C-S-H by the pozzolanic reaction and the filling of the void spaces both contribute to improved concrete quality, compressive and flexural strength, and long-term durability.
Mix Design with Pozzolans
Over the last 50 or more years, engineers and scientists have developed a number of strategies for effectively designing concrete with pozzolans. Depending on the type of pozzolan used and the design goals for the concrete, adjustments will be made to at least the cement replacement factor and the w/c ratio. Pozzolans such as VCAS™, blast furnace slag, and fly ash have relatively low water demands and can be used to replace up to 40% of cement in some concrete mixes. In contrast, silica fume has much finer particles with very high water demand, necessitating the use of high range water reducers or superplasticizers to even achieve a 10% cement replacement.
At the same time, the fine particle size renders this pozzolan more reactive, allowing concretes to achieve higher strengths more quickly. Like silica fume, metakaolin also has high water demand and is rarely used at replacement levels above 10%.
Another important consideration is that VCAS™ pozzolan has a specific gravity (SG) of 2.6 which is significantly lower than the SG 3.15 of Portland cement. This means that when VCAS™ is substituted for cement on an equal weight basis, there will be an increase in the paste volume. For a nominal 20wt% VCAS™ replacement of cement, this amounts to about a 4.2% increase in paste volume at fixed w/c ratio. This difference translates into an improved yield of cementious product and a mixture that is perceived to be more creamy and cohesive in consistency.
Depending on the reactivity of the pozzolan, the cement replacement factor, and the w/c ratio, the initial strength (up to 3 days) of concrete with pozzolans may be reduced by up to 20% compared to the strength of a control concrete without pozzolan. Between 14 and 28 days, however, the pozzolanic concretes typically have similar or higher strengths than the control concrete, with great benefit to the long-term durability, hardness, and strength of the concrete structure or component. High performance pozzolans, such as silica fume and metakaolin, typically have the least impact on the early strength. VCAS™ pozzolans fall into this high performance category and can reach or exceed the control strength by 3 days. Specific strength development data on the performance of VCAS™ pozzolans can be found in the VCAS™ Technology Data Sheets, and Technical Bulletins.
VCAS™ pozzolans are comparable in reactivity with silica fume and metakaolin, and are engineered to achieve their performance both by uniform materials chemistry and quenching and by fine particle size. As such, VCAS™ pozzolan have low surface area and smooth surfaces that have 10% less water demand than silica fume or metakaolin. This allows VCAS™ pozzolans to be used at cement replacement rates up to 30% or higher. Low water demand, high reactivity, and white color after cure are the hallmarks of an excellent pozzolan for white Portland cement concrete.
Benefits of Using Pozzolans
The following are benefits that are generally obtained from using pozzolans in concrete.
Workability: As they are replacing various percentages of Portland cement, pozzolans can have a significant impact — both positive and negative — on the workability of a concrete mix. Low water demand pozzolans, such as VCAS™, blast furnace slag and fly ash, generally increase workability at a given w/c ratio. In contrast, high surface area pozzolans such as silica fume and metakaolin cause a large reduction in the fluidity of the concrete, which necessitates the use of costly chemical admixtures (high range water reducers, superplasticizers) to make them workable. Even then, these types of concrete tend to be sticky and difficult to finish. VCAS™ pozzolans fall into the category of low water demand pozzolans.
Reduced Heat of Hydration: Historically, one of the most important reasons for including pozzolans in concrete was to lower the heat of hydration of the Portland cement in order to reduce thermally induced stress cracking. This is an especially important factor in massive concrete structures such as dams, tunnels, bridges, and large precast elements.
Increased Durability: Properly designed concretes made with pozzolans are generally stronger, harder and more durable. The physical densification of the cement matrix, coupled with the consumption of calcium hydroxide and cement alkalis brings about the following improvements in durability in pozzolanic concretes: greater resistance to sulfate attack; reduced alkali aggregate reactivity; less susceptibility to corrosion of embedded steel by chloride ion intrusion; and reduced efflorescence.
Reduced Efflorescence: Pozzolans react with excess calcium hydroxide and cement alkalis, helping to prevent primary efflorescence. Once the concrete cures, the pozzolanic reaction described earlier causes the concrete to become denser and less permeable. This limits egress of moisture, and reduces the lime and alkalis available to migrate to the surface causing primary and secondary efflorescence. VCAS™ pozzolans are similarly very effective at controlling efflorescence, particularly in white or colored concretes where this phenomenon can be a serious problem.
Improved Form Detail: A high fineness, high performance pozzolan such as VCAS™-micronHS is effective at reducing bug holes and better reproducing form detail in precast gray concrete. VCAS™ pozzolans are very effective at improving the surface quality and detailing of precast and molded white cement concrete products such as artificial stone, stucco, cladding panels, pool mixes, and terrazzo.
Pozzolan Color: The vast majority of pozzolans, such as coal fly ash, iron ore blast furnace slag, and silica fume, are dark colored and will usually strongly discolor white cement. Over 14-million tons of these pozzolans are used in gray Portland cement. VCAS™ and metakaolin pozzolans can be used with white cement. Upon the initial mixing of the white concrete paste, VCAS™ pozzolan will be slightly blue tint, and metakaolin will be slightly buff tint. During the first seven days of curing, the white concrete will brighten as the pozzolan reacts, such that both pozzolan will be about as white as the white cement control. The VCAS™ blue tint, measured as lower B value, will often make the VCAS™ pozzolan appear whiter to the human eye than metakaolin.
Pozzolans and Sustainability
Pozzolans have an important role to play in sustainable “Green” construction by increasing service life and reducing the net greenhouse gas emissions (GHG) and energy consumption for a cubic yard of concrete. For every ton of cement replaced by VCAS™ pozzolans, there is a net reduction of about 1 ton of CO2emissions, which means that every 21-ton truckload used is equivalent to taking three automobiles off the road. In addition, the heat saved is 4.29 million BTU’s/ton, which would heat the average home for more than a week. In addition, every ton VCAS™ used saves 1.5 tons of virgin raw materials needed to make a ton of cement. VCAS™ pozzolans represent a high value recycling opportunity.
